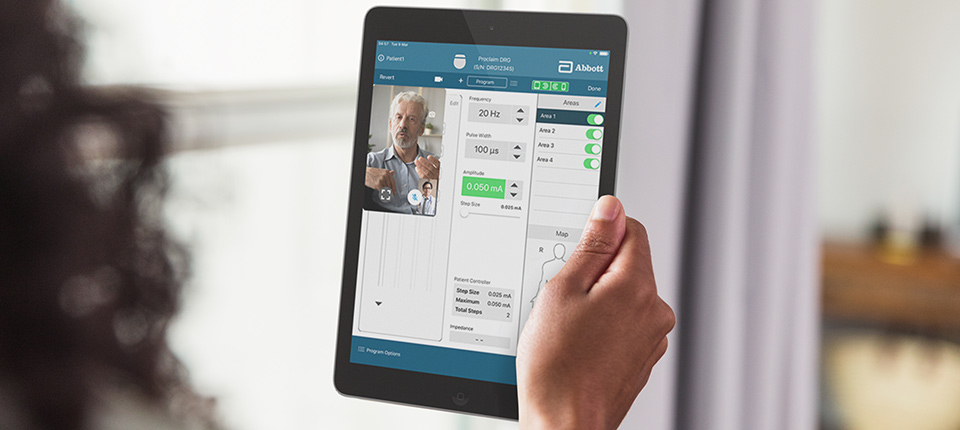
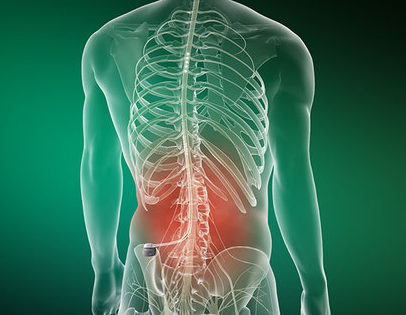
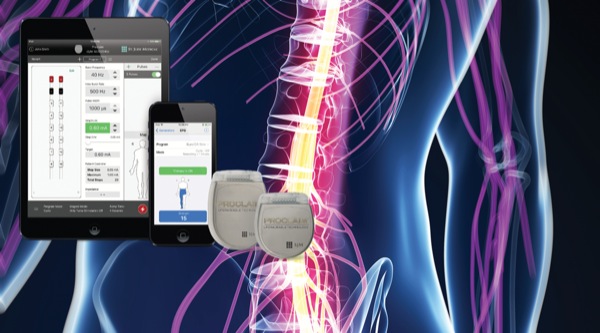
Proclaim™ XR Spinal Cord Stimulation (SCS) System with BurstDR™ Technology: Paddle Lead Implant Procedure | Intended to be viewed upon completion of a successful evaluation, this video illustrates the permanent implant procedure
New Data Reinforces Benefits Of Abbott's BurstDR Spinal Cord Stimulation For Chronic Pain | Orthopedic Design Technology
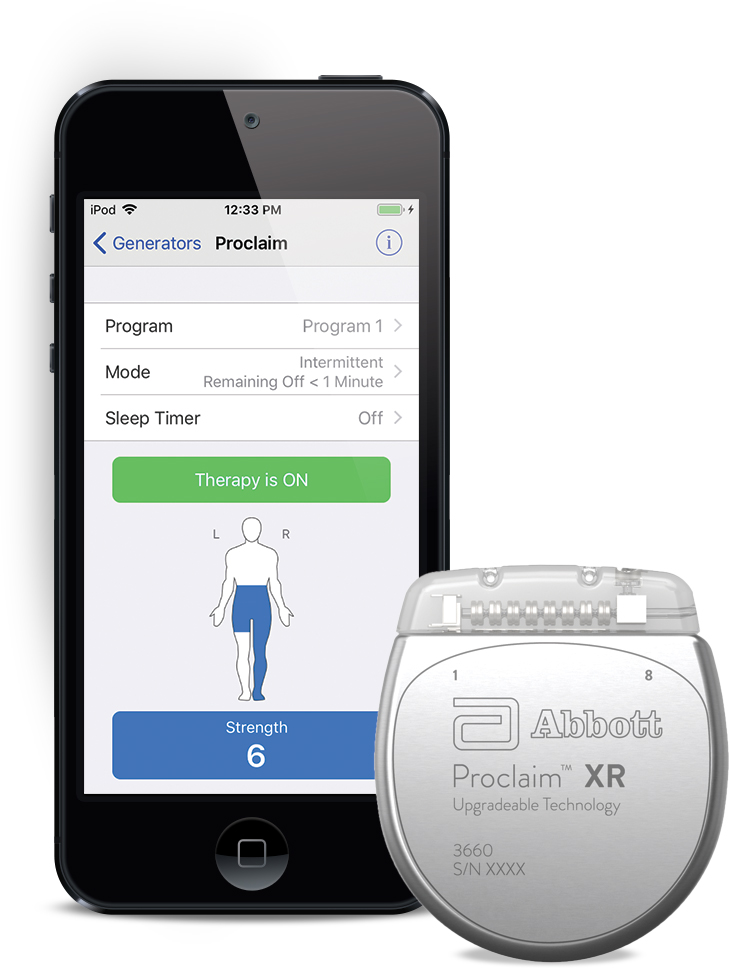
FDA Approves Abbott's "Low Dose," Recharge-Free Spinal Cord Stimulation System with up to Ten Year Battery Life* for People Living with Chronic Pain

Abbott launches Eterna spinal cord stimulation system for the treatment of chronic pain - Spinal News International
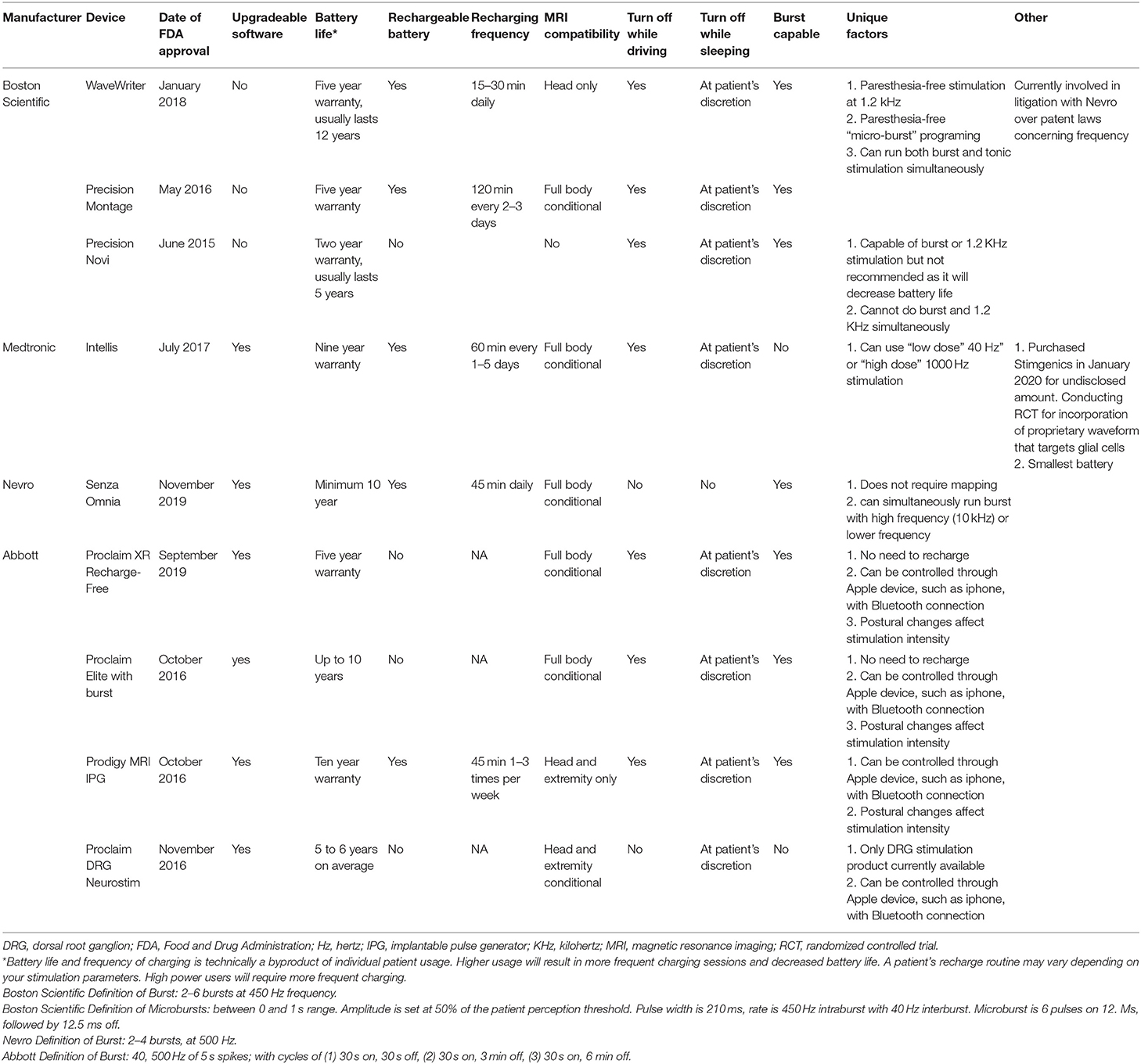
Frontiers | Survey of Spinal Cord Stimulation Hardware Currently Available for the Treatment of Chronic Pain in the United States

Spinal cord stimulation for the treatment of peripheral neuropathic pain | Tidsskrift for Den norske legeforening
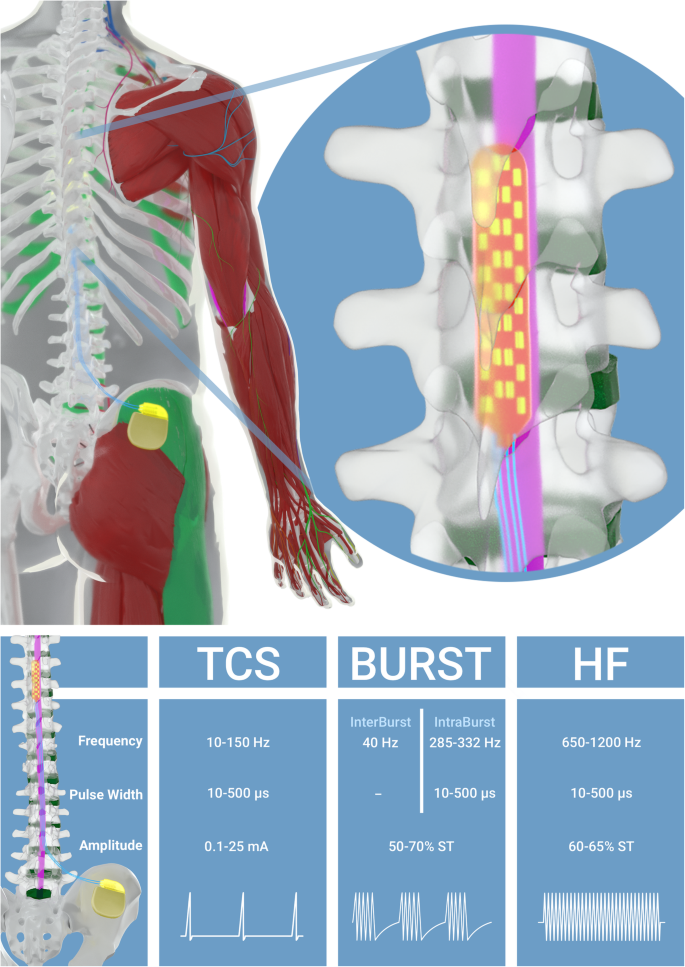
Comparison of conventional, burst and high-frequency spinal cord stimulation on pain relief in refractory failed back surgery syndrome patients: study protocol for a prospective randomized double-blinded cross-over trial (MULTIWAVE study) | Trials